IntroductionI haven't posted in a while because I have been trying to find new and exciting papers a little out of my comfort zone. And it just so happened that Scripps hosted National Academy member, Diane Newman, this week for a special seminar sponsored by the graduate students in the Biotechnology Training Fellowship. Almost all of my interest has been driven by a graduate student in my current lab, Doug Sweeney, who is actively looking at the mechanisms of electron transfer in marine sediment bacterium. If you are interested in any of these topics, definitely feel free to email him!
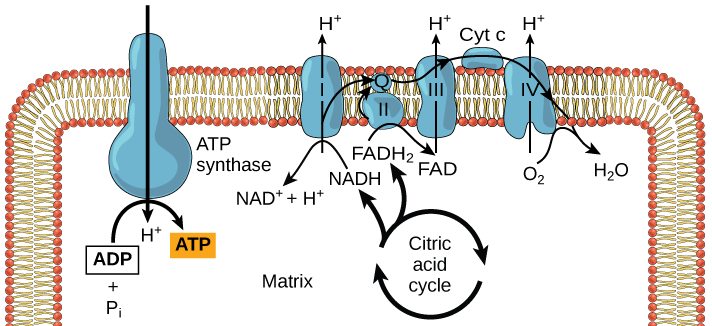
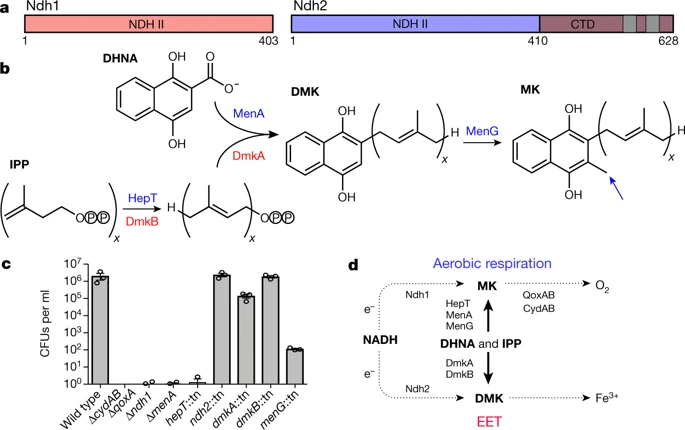
Redox potential are everything in microbial communitiesBefore I jump into the chemistry side of things, I first wanted to give a brief overview of why electron transfer is so imperative. Most of you probably know this, but this process of transferring electrons enables cells to maintain cellular function and generate energy for additional processes. But since I am not a chemist, I obviously want to take a step back very briefly to highlight how important these processes can be at multiple scales, especially when we consider entire communities! A recent paper by Ramirez-Flandes et al. (2019) assessed metagenomes from 18 biomes to determine what distinguishing characteristics delineate microbial communities [1]. Most of us are aware that taxonomic signatures can illuminate certain microbial biomes (e.g., Cyanobacteria are usually associated with marine samples). However, this paper decided to turn to functional characterization to differentiate various microbial biomes. What they found was that oxidoreductase gene profiles separated the biomes into three main clusters: anoxic biomes (e.g., host-associated and marine sediments), aquatic biomes, and soil-associated biomes. Within the oxidoreductase gene profiles, different biomes were associated with various groups of oxidoreductases related to substrate availability and stress conditions. Redox processes alone delineate microbial communities! Why are redox processes so important?I started with this first paper because it nicely outline that various biomes present various challenges for the bacteria that reside there. For example, in the absence of oxygen, how do organisms maintain their cellular processes? One way is fermentation, but this leads to toxic byproducts and is not a sustainable option. Another is the use of alternative electron acceptors. In soils, sediments, host-associated, microbes are constantly dealing with anoxic conditions and yet we find traditionally described aerobic organisms. Some organisms have adapted to utilize redox-active minerals that are abundant in certain biomes, like iron or manganese in soils. This is absolutely fascinating, microbes have evolved novel strategies to exchange electrons with minerals by transferring electrons from the cytosol to the exterior of the cell [for more see this review [2]]. So how do these microbes transfer electrons outside the cell? The above image [3] depicts the beautiful pigments bacteria are capable of producing on a plate that most of us take for granted. But what is the role of these pigments? Some may be related to UV or desiccation, but others often change colors which is indicative of a redox reaction. This led to the topic of the entire post, the use of these molecules as extracellular electron shuttles. Some known shuttles are the natural products, quinones, phenazines, and flavins, which shuttle electrons to an acceptor. Glasser et al. [3] found that phenazines in particular are widespread along with the transcription factor, SoxR, that senses redox-active metabolites. From Dr. Newman's seminar this week, she discussed (in fascinating detail) how these phenazines in biofilms interact with eDNA (or dead cell DNA) to stabilize redox reactions and allow for the anaerobic survival of the producing organisms to maintain cellular viability. These discoveries really revolutionize our understanding of microbial metabolism. But the biggest question I always had is the production of such natural products must be expensive, especially considering their role is essential in stressed conditions. And even if production is sustained, how can the cell ensure an adequate return on its investment; in other words, how can the extracellular molecule not be lost to the environment. Dr. Newman presented an amazing solution to this problem by addressing shuttle diffusion gradients, electron hopping, and more [see her review [3] and stay tuned for some forthcoming work from her lab]. Why do microbes go through all this trouble???Finally, this question brings me to the Journal Club paper of the week [4]. Why are microbes evolving these intricate solutions to a seemingly simple problem of where to deposit electrons. As a reminder, the entire point of transferring electrons is to generate ATP, but to do so requires the use of electron shuttles such as NADH to carry electrons from one reaction to another. This essential molecule then can be used as a reducing agent to donate electrons and become oxidized as NAD+, ready to accept more electrons and continue the cycle. In the absence of a terminal electron acceptor, such as oxygen, NADH will build up in the cell. What would be a good strategy to deal with NADH buildup? Well in a paper by Light et al. [4] the authors identified a parallel electron transfer pathway in anaerobic conditions in the pathogen Listeria monocytogenes. In the presence of oxygen, normal aerobic respiration occurs with an essential enzyme for metabolism, NADH dehydrogenase (Ndh1 in above figure), catalyzing electron exchange to a quinone derivative, the first step in the electron transport chain. Under anaerobic conditions, this process cannot continue as NADH cannot be recycled. So, a parallel solution evolved using another NADH dehydrogenase (Ndh2 in above figure) enables NAD+ concentrations to be restored. Using transposon mutants the authors show cell viability using knockouts of these essential genes (panel C); for instance, in the presence of oxygen the knockout of Ndh2 (or any other genes in the anaerobic pathway) does not inhibit cellular growth. Under anaerobic conditions, the demethylated quinone derivative (DMK) accepts electrons from NADH via Ndh2. The authors very thoroughly outline the subsequent electron transferring system where DMK donates electrons to membrane proteins and finally to environmental flavins to shuttle electrons and finally to extracellular acceptors, like Fe(III). More and more, it appears that these innovative strategies to deal with electron transfer are a fundamental issue in all microbial life, not limited to specialized processes in mineral-respiring bacteria. All bacteria require cell viability and processes. These processes are driven entirely by redox potential which is mediated by electron shuttles. This really blows my mind as I have never considered the magnitude of the electron transport chain (and the various innovations) to deal with the recycling of electron shuttles. Papers:1. Ramirez-Flandes S, Gonzalez B, Ulloa O. (2019). Redox traits characterize the organization of global microbial communities. Proceedings to the National Academy of Sciences 116(9): 3630-3635.
2. Shi L, Dong H, Reguera G, Beyenal H, Lu A, Liu J, Yu HQ, Frederickson JK. (2016). Extracellular electron transfer mechanisms between microorganisms and minerals. Nature Reviews Microbiology 14: 651-662. 3. Glasser NR, Saunders SH, Newman DK. (2017). The colorful world of extracellular electron shuttles. Annual Review of Microbiology 71: 731-751. 4. Light SH, Su L, Rivera-Lugo R, Cornejo JA, Louie A, Iaverone AT, Ajo-Franklin CM, Portnoy DA. (2018). A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 562: 140-144.
0 Comments
Leave a Reply. |
AuthorSome thoughts on some (small) things Archives
May 2023
Categories |
Proudly powered by Weebly
 RSS Feed
RSS Feed